Treatment
How to treat Mesothelioma? The prognosis for malignant mesothelioma remains disappointing,
although there have been some modest improvements in prognosis from
newer chemotherapies and multimodality treatments.
[29]
Treatment of malignant mesothelioma at earlier stages has a better
prognosis, but cures are exceedingly rare. Clinical behavior of the
malignancy is affected by several factors including the continuous
mesothelial surface of the pleural cavity which favors local metastasis
via exfoliated cells, invasion to underlying tissue and other organs
within the pleural cavity, and the extremely long latency period between
asbestos exposure and development of the disease. The histological
subtype and the patient's age and health status also help predict
prognosis. The epithelioid histology responds better to treatment and
has a survival advantage over sarcomatoid histology.
[30]
Surgery
Surgery, by itself, has proved disappointing. In one large series, the median survival with surgery (including extrapleural
pneumonectomy) was only 11.7 months.
[29]
However, research indicates varied success when used in combination
with radiation and chemotherapy (Duke, 2008). (For more information on
multimodality therapy with surgery, see below). A
pleurectomy/decortication is the most common surgery, in which the
lining of the chest is removed. Less common is an extrapleural
pneumonectomy (EPP), in which the lung, lining of the inside of the
chest, the hemi-
diaphragm and the
pericardium are removed.
Radiation
For patients with localized disease, and who can tolerate a radical
surgery, radiation is often given post-operatively as a consolidative
treatment. The entire hemi-thorax is treated with radiation therapy,
often given simultaneously with chemotherapy. Delivering radiation and
chemotherapy after a radical surgery has led to extended life expectancy
in selected patient populations with some patients surviving more than 5
years. As part of a curative approach to mesothelioma, radiotherapy is
also commonly applied to the sites of
chest drain insertion, in order to prevent growth of the tumor along the track in the chest wall.
Although mesothelioma is generally resistant to curative treatment with
radiotherapy
alone, palliative treatment regimens are sometimes used to relieve
symptoms arising from tumor growth, such as obstruction of a major blood
vessel. Radiation therapy when given alone with curative intent has
never been shown to improve survival from mesothelioma. The necessary
radiation dose to treat mesothelioma that has not been surgically
removed would be very toxic.
Chemotherapy
Chemotherapy is the only treatment for mesothelioma that has been
proven to improve survival in randomised and controlled trials. The
landmark study published in 2003 by Vogelzang and colleagues compared
cisplatin chemotherapy alone with a combination of cisplatin and
pemetrexed
(brand name Alimta) chemotherapy in patients who had not received
chemotherapy for malignant pleural mesothelioma previously and were not
candidates for more aggressive "curative" surgery.
[31]
This trial was the first to report a survival advantage from
chemotherapy in malignant pleural mesothelioma, showing a statistically
significant improvement in
median
survival from 10 months in the patients treated with cisplatin alone to
13.3 months in the group of patients treated with cisplatin in the
combination with pemetrexed and who also received supplementation with
folate and vitamin B
12. Vitamin supplementation was given to
most patients in the trial and pemetrexed related side effects were
significantly less in patients receiving pemetrexed when they also
received daily oral folate 500mcg and intramuscular vitamin B
12
1000mcg every 9 weeks compared with patients receiving pemetrexed
without vitamin supplementation. The objective response rate increased
from 20% in the cisplatin group to 46% in the combination pemetrexed
group. Some side effects such as nausea and vomiting,
stomatitis,
and diarrhoea were more common in the combination pemetrexed group but
only affected a minority of patients and overall the combination of
pemetrexed and cisplatin was well tolerated when patients received
vitamin supplementation; both
quality of life and
lung function tests improved in the combination pemetrexed group. In February 2004, the United States
Food and Drug Administration
approved pemetrexed for treatment of malignant pleural mesothelioma.
However, there are still unanswered questions about the optimal use of
chemotherapy, including when to start treatment, and the optimal number
of cycles to give.
Cisplatin in combination with
raltitrexed
has shown an improvement in survival similar to that reported for
pemetrexed in combination with cisplatin, but raltitrexed is no longer
commercially available for this indication. For patients unable to
tolerate pemetrexed, cisplatin in combination with gemcitabine or
vinorelbine is an alternative, or vinorelbine on its own, although a
survival benefit has not been shown for these drugs. For patients in
whom cisplatin cannot be used, carboplatin can be substituted but
non-randomised data have shown lower response rates and high rates of
haematological toxicity for carboplatin-based combinations, albeit with
similar survival figures to patients receiving cisplatin.
[32]
In January 2009, the United States FDA approved using conventional
therapies such as surgery in combination with radiation and or
chemotherapy on stage I or II Mesothelioma after research conducted by a
nationwide study by Duke University concluded an almost 50 point
increase in remission rates.
Immunotherapy
Treatment regimens involving immunotherapy have yielded variable results. For example, intrapleural inoculation of
Bacillus Calmette-Guérin
(BCG) in an attempt to boost the immune response, was found to be of no
benefit to the patient (while it may benefit patients with
bladder cancer). Mesothelioma cells proved susceptible to in vitro lysis by LAK cells following activation by
interleukin-2
(IL-2), but patients undergoing this particular therapy experienced
major side effects. Indeed, this trial was suspended in view of the
unacceptably high levels of IL-2 toxicity and the severity of side
effects such as fever and cachexia. Nonetheless, other trials involving
interferon alpha have proved more encouraging with 20% of patients
experiencing a greater than 50% reduction in tumor mass combined with
minimal side effects.
Heated intraoperative intraperitoneal chemotherapy
A procedure known as heated intraoperative intraperitoneal
chemotherapy was developed by Paul Sugarbaker at the Washington Cancer
Institute.
[33]
The surgeon removes as much of the tumor as possible followed by the
direct administration of a chemotherapy agent, heated to between 40 and
48°C, in the abdomen. The fluid is perfused for 60 to 120 minutes and
then drained.
This technique permits the administration of high concentrations of
selected drugs into the abdominal and pelvic surfaces. Heating the
chemotherapy treatment increases the penetration of the drugs into
tissues. Also, heating itself damages the malignant cells more than the
normal cells.
This technique is also used in patients with malignant pleural mesothelioma.
[34]
Multimodality therapy
All of the standard approaches to treating solid tumors—radiation,
chemotherapy, and surgery—have been investigated in patients with
malignant pleural mesothelioma. Although surgery, by itself, is not very
effective, surgery combined with adjuvant chemotherapy and radiation
(trimodality therapy) has produced significant survival extension (3–14
years) among patients with favorable prognostic factors.
[35]
However, other large series of examining multimodality treatment have
only demonstrated modest improvement in survival (median survival 14.5
months and only 29.6% surviving 2 years).
[29]
Reducing the bulk of the tumor with cytoreductive surgery is key to
extending survival. Two surgeries have been developed: extrapleural
pneumonectomy and pleurectomy/decortication. The indications for
performing these operations are unique. The choice of operation depends
on the size of the patient's tumor. This is an important consideration
because tumor volume has been identified as a prognostic factor in
mesothelioma.
[36]
Pleurectomy/decortication spares the underlying lung and is performed
in patients with early stage disease when the intention is to remove all
gross visible tumor (macroscopic complete resection), not simply
palliation.
[37]
Extrapleural pneumonectomy is a more extensive operation that involves
resection of the parietal and visceral pleurae, underlying lung,
ipsilateral diaphragm, and ipsilateral pericardium. This operation is
indicated for a subset of patients with more advanced tumors, who can
tolerate a pneumonectomy.
[38]
Epidemiology
Although reported incidence rates have increased in the past 20
years, mesothelioma is still a relatively rare cancer. The incidence
rate varies from one country to another, from a low rate of less than 1
per 1,000,000 in Tunisia and Morocco, to the highest rate in Britain,
Australia and Belgium: 30 per 1,000,000 per year.
[39] For comparison, populations with high levels of smoking can have a
lung cancer
incidence of over 1,000 per 1,000,000. Incidence of malignant
mesothelioma currently ranges from about 7 to 40 per 1,000,000 in
industrialized Western nations, depending on the amount of asbestos
exposure of the populations during the past several decades.
[40]
It has been estimated that incidence may have peaked at 15 per
1,000,000 in the United States in 2004. Incidence is expected to
continue increasing in other parts of the world. Mesothelioma occurs
more often in men than in women and risk increases with age, but this
disease can appear in either men or women at any age. Approximately one
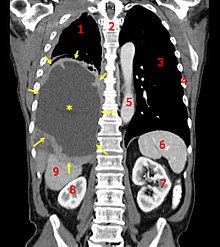
fifth to one third of all mesotheliomas are peritoneal.
Between 1940 and 1979, approximately 27.5 million people were occupationally exposed to asbestos in the United States.
[41]
Between 1973 and 1984, the incidence of pleural mesothelioma among
Caucasian males increased 300%. From 1980 to the late 1990s, the death
rate from mesothelioma in the USA increased from 2,000 per year to
3,000, with men four times more likely to acquire it than women.
The incidence of peritoneal mesothelioma is 0.5–3.0 per million per year in men, and 0.2–2.0 per million per year in women.
[